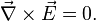Electrostatics
From Wikipedia, the free encyclopedia
| Electromagnetism | |
| Electricity · Magnetism | |
| Electrostatics | |
|---|---|
| Electric charge | |
| Coulomb's law | |
| Electric field | |
| Gauss's law | |
| Electric potential | |
| Electric dipole moment | |
| Magnetostatics | |
| Ampère's circuital law | |
| Magnetic field | |
| Magnetic flux | |
| Biot-Savart law | |
| Magnetic dipole moment | |
| Electrodynamics | |
| Electrical current | |
| Lorentz force law | |
| Electromotive force | |
| (EM) Electromagnetic induction | |
| Faraday-Lenz law | |
| Displacement current | |
| Maxwell's equations | |
| (EMF) Electromagnetic field | |
| (EM) Electromagnetic radiation | |
| Electrical Network | |
| Electrical conduction | |
| Electrical resistance | |
| Capacitance | |
| Inductance | |
| Impedance | |
| Resonant cavities | |
| Waveguides | |
| Tensors in Relativity | |
| Electromagnetic tensor | |
| Electromagnetic stress-energy tensor | |
Electrostatics (also known as static electricity) is the branch of physics that deals with the phenomena arising from what seem to be stationary electric charges. This includes phenomena as simple as the attraction of plastic wrap to your hand after you remove it from a package to apparently spontaneous explosion of grain silos, to damage of electronic components during manufacturing, to the operation of photocopiers. Electrostatics involves the buildup of charge on the surface of objects due to contact with other surfaces. Although charge exchange happens whenever any two surfaces contact and separate, the effects of charge exchange are usually only noticed when at least one of the surfaces has a high resistance to electrical flow. This is because the charges that transfer to or from the highly resistive surface are more or less trapped there for a long enough time for their effects to be observed. These charges then remain on the object until they either bleed off to ground or are quickly neutralized by a discharge: e.g., the familiar phenomenon of a static 'shock' is caused by the neutralization of charge built up in the body from contact with nonconductive surfaces.
Contents
|
[edit] The electrostatic approximation
The validity of the electrostatic approximation rests on the assumption that the electric field is irrotational:
From Faraday's law, this assumption implies the absence or near-absence of time-varying magnetic fields:
In other words, electrostatics does not require the absence of magnetic fields or electric currents. Rather, if magnetic fields or electric currents do exist, they must not change with time, or in the worst-case, they must change with time only very slowly. In some problems, both electrostatics and magnetostatics may be required for accurate predictions, but the coupling between the two can still be ignored.
[edit] Electrostatic potential
Because the electric field is irrotational, it is possible to express the electric field as the gradient of a scalar function, called the electrostatic potential (also known as the voltage). Thus, the electrostatic potential φ is related to the electric field E by the equation
[edit] Fundamental concepts
[edit] Coulomb's law
The fundamental equation of electrostatics is Coulomb's law, which describes the force between two point charges Q1 and Q2:
[edit] The electric field
The electric field (in units of volts per meter) is defined as the force (in newtons) per unit charge (in coulombs). From this definition and Coulomb's law, it follows that the magnitude of the electric field E created by a single point charge Q is
[edit] Gauss's law
Gauss' law states that "the total electric flux through a closed surface is proportional to the total electric charge enclosed within the surface". The constant of proportionality is the permittivity of free space.
Mathematically, Gauss's law takes the form of an integral equation:
Alternatively, in differential form, the equation becomes
[edit] Poisson's equation
The definition of electrostatic potential, combined with the differential form of Gauss's law (above), provides a relationship between the potential φ and the charge density ρ:
This relationship is a form of Poisson's equation.
[edit] Laplace's equation
In the absence of unpaired electric charge, the equation becomes
which is Laplace's equation.
[edit] Triboelectric series
The triboelectric effect is a type of contact electrification in which certain materials become electrically charged when coming into contact with another, different, material, and are then separated. The polarity and strength of the charges produced differ according to the materials, surface roughness, temperature, strain, and other properties. It is therefore not very predictable, and only broad generalizations can be made. Amber, for example, can acquire an electric charge by friction with a material like wool. This property, first recorded by Thales of Miletus, suggested the word "electricity", from the Greek word for amber, èlectròn. Other examples of materials that can acquire a significant charge when rubbed together include glass rubbed with silk, and hard rubber rubbed with fur.
[edit] Electrostatic generators
The presence of surface charge imbalance means that the objects will exhibit attractive or repulsive forces. This surface charge imbalance, which yields static electricity, can be generated by touching two differing surfaces together and then separating them due to the phenomena of contact electrification and the triboelectric effect. Rubbing two nonconductive objects generates a great amount of static electricity. This is not just the result of friction; two nonconductive surfaces can become charged by just being placed one on top of the other. Since most surfaces have a rough texture, it takes longer to achieve charging through contact than through rubbing. Rubbing objects together increases amount of adhesive contact between the two surfaces. Usually insulators, e.g., substances that do not conduct electricity, are good at both generating, and holding, a surface charge. Some examples of these substances are rubber, plastic, glass, and pith. Conductive objects only rarely generate charge imbalance except, for example, when a metal surface is impacted by solid or liquid nonconductors. The charge that is transferred during contact electrification is stored on the surface of each object. Static electric generators, devices which produce very high voltage at very low current and used for classroom physics demonstrations, rely on this effect.
Note that the presence of electric current does not detract from the electrostatic forces nor from the sparking, from the corona discharge, or other phenomena. Both phenomena can exist simultaneously in the same system.
- See also: Friction machines, Wimshurst machines, and Van de Graaf generators.
[edit] Charge neutralisation
Natural electrostatic phenomena are most familiar as an occasional annoyance in seasons of low humidity, but can be destructive and harmful in some situations (e.g. electronics manufacturing). When working in direct contact with integrated circuit electronics (especially delicate MOSFETs), or in the presence of flammable gas, care must be taken to avoid accumulating and suddenly discharging a static charge (see electrostatic discharge).
[edit] Charge induction
Charge induction occurs when a negatively charged object repels electrons from the surface of another object, leaving it positively charged. An attractive force is then exerted by the objects. For example, when a balloon is rubbed, the balloon will stick to the wall as an attractive force is exerted by two oppositely charged surfaces (the surface of the wall gains an electric charge due to charge induction, as the free electrons at the surface of the wall are repelled by the negative balloon, creating a positive wall surface, which is subsequently attracted to the surface of the balloon).
[edit] 'Static' electricity
| The tone or style of this article or section may not be appropriate for Wikipedia. Specific concerns may be found on the talk page. See Wikipedia's guide to writing better articles for suggestions. |
Before the year 1832, when Michael Faraday published the results of his experiment on the identity of electricities, physicists thought "static electricity" was somehow different from other electrical charges. Michael Faraday proved that the electricity induced from the magnet, voltaic electricity produced by a battery, and static electricity were all the same.
Static electricity is usually caused when certain materials are rubbed against each other, like wool on plastic or the soles of shoes on carpet. The process causes electrons to be pulled from the surface of one material and relocated on the surface of the other material.
A static shock occurs when the surface of the second material, negatively charged with electrons, touches a positively-charged conductor.
Static electricity is commonly used in xerography, air filters, and some automotive paints. Static Electricity is a build up of electric charges on two objects that have become separated from each other.
[edit] Static electricity and chemical industry
| This section may require cleanup to meet Wikipedia's quality standards. Please discuss this issue on the talk page, and/or replace this tag with a more specific message. Editing help is available. This section has been tagged since September 2007. |
When dissimilar materials come together and are then taken apart, separation and accumulation of electric charge takes place. This leads to the phenomenon of static electricity. The mild shock that one gets on touching certain surfaces is a result of the discharge of the built-up charge through the body. Static electricity maybe fun to experiment with in school laboratories, but is a high potential hazard in the chemical industry. The problem comes from the spontaneous and uncontrolled discharge of the charges, which have adequate energy to ignite an explosive mixture.
The ability of a fluid to retain an electrostatic charge depends on its electrical conductivity. Fluids which have electric conductivity below 50 pico siemens /cm (pico siemens /cm is the unit for measurement of electrical conductivity, also known sometimes as CU, Conductivity Units) are called accumulators, and those having values above 50 pico siemens /cm are non-accumulators. In non-accumulators, charges combine as fast as they are separated and hence electrostatic charge generation is not significant. 50 pico siemens /cm is the recommended minimum value of electrical conductivity for adequate removal of charge from a fluid.
Another important term is relaxation time, which is calculated as 18 divided by electrical conductivity. Thus a fluid that has an electrical conductivity of 1 pico siemens /cm has a relaxation time of 18 seconds. 4 to 5 times the relaxation time is the time required to be given to a fluid for the charges to dissipate itself. 90 seconds for the fluid in this example.
Flow of poorly conducting hydrocarbon liquids at high velocities in pipelines is an important source of generation of static charges. This is more so with pipe diameters larger than 8 inches and not so much with smaller diameter pipelines. Static charge generation in such flow systems is best controlled by limiting the velocity. The British standard BS PD CLC/TR 50404:2003 (formerly BS-5958-Part 2) Code of Practice for Control of Undesirable Static Electricity prescribes velocity limits. The presence of water in the hydrocarbon makes a big difference and in this case the velocity should be limited to 1 m/sec.
Bonding and earthing are the usual ways by which charge separation can be prevented. For fluids with electrical conductivity below 10 pico siemens/cm, bonding and earthing are not adequate for charge dissipation. Antistatic additives would be required in this case.
Applicable Standards
1.BS PD CLC/TR 50404:2003 Code of Practice for Control of Undesirable Static Electricity
2.NFPA 77 (2007) Recommended Practice on Static Electricity
3.API RP 2003 (1998) Protection Against Ignitions Arising Out of Static, Lightning, and Stray Currents