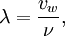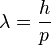Wavelength
From Wikipedia, the free encyclopedia
In physics, wavelength is the distance between repeating units of a propagating wave of a given frequency. It is commonly designated by the Greek letter lambda (λ). Examples of wave-like phenonomena are light, water waves, and sound waves.
In a wave, a property varies with the position. For example, this property can be the air pressure for a sound wave, or the magnitude of the electric or the magnetic field for light. The wavelengths of frequencies audible to the human ear (20 Hz – 20 kHz) are between approximately 17 m and 17 mm, respectively. Visible light ranges from deep red, roughly 700 nm to violet, roughly 400 nm (430--750 THz). For other examples, see electromagnetic spectrum.
Contents
|
[edit] Relationship with frequency
Wavelength λ is inverse proportional with the frequency ν (Greek "nu"), the number of wave periods per time unit passing a given point, as in
where vw is the propagation velocity of the wave. In the case of electromagnetic radiation, such as light, in a vacuum, this speed is the speed of light, 299,792,458 m/s or about 109 km/h. For sound waves in air, this is the speed of sound, 344 m/s (1238 km/h) in air at room temperature. Usually, SI units are used, where the wavelength is expressed in meters, the frequency in Hz, and the propagation velocity in meters per second.
[edit] In non-vacuum media
The speed of light in most media is lower than in vacuum, which means that the same frequency will correspond to a shorter wavelength in the medium than in vacuum. The wavelength in the medium is
where n is the refractive index of the medium. Wavelengths of electromagnetic radiation are usually quoted in terms of the vacuum wavelength, unless specifically indicated as the "wavelength in the medium".
[edit] De Broglie wavelength of particles
Louis de Broglie postulated that all particles with momentum have a wavelength
where h is Planck's constant, and p is the momentum of the particle. This hypothesis was at the basis of quantum mechanics. Nowadays, this wavelength is called the de Broglie wavelength. For example, the electrons in a CRT display have a De Broglie wavelength of about 10-13 m.